|
Participant Update: Summer 2013 |
|
|
| U-BIOPRED consortium members at the 2013 annual meeting |
|
 |
|
| U-BIOPRED: A summary for participants |
|
|
We are now over halfway through the U-BIOPRED project, with two more years to go and have arrived to the target number of 1025 participants.
We would like to thank you and all the people who volunteered to take part. Here is a summary of what has been going on in the project, what has happened to the samples you gave, and what has been achieved so far. |
|
|
|
| Making a complex project work |
|
The U-BIOPRED project brings together the expertise of academia and industry partners, to improve the diagnosis of asthma. U-BIOPRED also has 10 partners from medical industries, which develop and produce medications, and seventeen treatment centres cooperating in the U-BIOPRED study. |
Together they identified 11 different ways to look at the data from all the participants. U-BIOPRED has a biobank where over 40,000 samples, given by participants, are stored and ready for analysis. National and European patient organisations, scientific societies and individual patients with asthma are involved as partners, to ensure that the needs of people with asthma and the participants remain the focus of the project. |
|
|
|
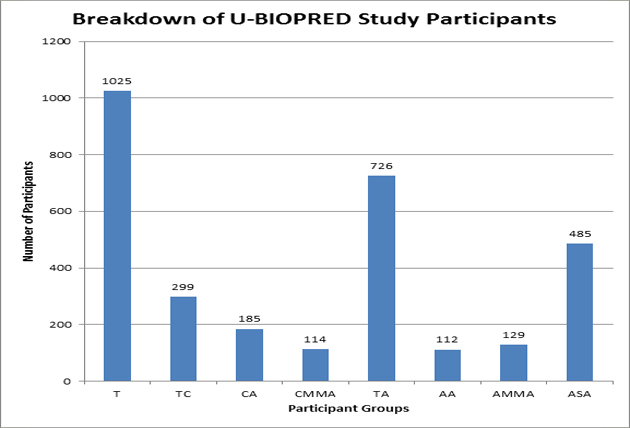 |
U-BIOPRED needed a large number of participants to be scientifically reliable and with 1025 recruitments it is one of the largest projects of its kind. People with and without asthma have volunteered to take part in the project, providing samples and other information about their general health and asthma. There are 726 adults and 299 children (paediatric) being studied. Reaching this number of participants shows how important it is to the people who took part to find out more about asthma by getting involved in research projects. |
Guide to Breakdown of U-BIOPRED Study Participants Graph
T - Total participants recruited
TC - Total Children
CA - Children with Asthma (school age and pre-school)
CMMA - Children with Mild-Moderate Asthma (school age and pre-school)
TA - Total Adults
AA - Adults without Asthma
AMMA - Adults with Mild-Moderate Asthma
ASA - Adults with Severe Asthma (smokers & non-smokers) |
|
|
| What is happening to your data? |
|
By looking closely at the samples and measurements that you and the other volunteers gave, we can uncover the different characteristics and types of severe asthma, a process known as phenotyping. Samples of blood, urine, phlegm from the airways (sputum), and in some cases small pieces of lung tissue, were collected. These samples are being stored in the U-BIOPRED biobank. |
When one of the projectís research centres needs to analyse a sample they have to request the sample from the biobank in the UK. The sample is then safely transported to the research centre and returned as soon as the tests have been performed. Different centres specialize in different types of tests, which is why the samples are all held in one place and sent out when required. |
|
|
|
Discovering different types of asthma groups |
|
In the first tests with the urine and sputum samples of 60 adults, we looked at different types of fats (lipids). |
We expected the composition of lipids to differ between the different types of asthma; for example, severe asthma can have different levels of lipids from people with mild asthma. The results proved that different groups of people can be identified by lipid compositions.
|
|
|
| Involving patient groups as partners |
|
Four patient organisations (Longfonds, Asthma UK, EFA and Lega Italiano Anti Fumo) and the European Lung Foundation (ELF) are partnering the project.
They ensure that patients have been actively involved from the beginning of the project. These organisations and patient members help communicate the project and its results to the public help solve issues such as recruiting enough participants, identifying and helping resolve ethical and patient safety issues, and advise on the performance of the experiments and how to use the results in future studies. Different boards within the project address these issues. |
Visit our website to find out more:
www.ubiopred.european-lung-foundation.org/advisoryboards
European Lung Foundation - www.european-lung-foundation.org
Find out more about the patient organistions:
Longfonds - www.longfonds.nl
Asthma UK - www.asthma.org.uk
EFA - www.efanet.org
Lega Italiana Antifumo - www.liaf-onlus.org |
|
|
|
Keeping in touch with scientists and the public |
|
A recent survey commissioned by our funder, the Innovative Medicines Initiative (IMI), showed that U-BIOPRED is also successfully raising awareness in academic centres and to the public. So far partners of U-BIOPRED have made several presentations at the leading lung health conferences and meetings with the public. |
Using Twitter (https://twitter.com/UBIOPRED) and LinkedIn (http://goo.gl/WpqDD) has helped raise our profile as well as being a useful resource for participants and other people involved in the study to stay informed and communicate with us. A factsheet on bronchoscopy was created to help explain the procedure and give the patientsí perspective to encourage participants to give a sample. This factsheet can be downloaded from the U-BIOPRED website. |
|
|
| The expectations of the project |
|
|
Several researchers and patients involved shared their thoughts on the project and expectations for its impact on people with asthma. Here are a few of their comments:

'I hope that we can find the handprints of severe asthma which link the biological and clinical information. If we can do this we can give real insight into asthma treatment therapy by finding the right drugs to treat each individual person with asthma.'
Xian Yang, Bioinformatic data researcher
'We hope through implementation of the systems approach to better understand what happens during exacerbations, thus helping each patient to manage their asthma and reach a more controlled state, whether they have mild, moderate or severe asthma.'
Charles Auffrey, Systems biology
|
 'By working with the pharmaceutical companies and teaching them these new scientific methodologies and approaches to the treatment of asthma, they will be better equipped to engineer specific treatments for specific groups of severe asthma, so this project will provide the instruments for improved medical practice'
Peter Sterk, project coordinator
'[The research] will help to find better treatment options, save money and increase patientsí trust in medicine. I hope that we can learn lessons from the project, such as what was successful, what wasnít so successful, and how we can better use patientsí opinions and advice in the future.'
Lina Buzermaniene, Patient input |
'Co-ordinating the various views and experiences of the public and project members can take a lot of time and effort, but it is worth it as it focuses the project on its main goal, bringing us closer to discovering new and improved treatments - sooner.'
Malayka Rahman, Patient organisations
'It is really important to think about what happens after U-BIOPRED to ensure that all those samples collected really are put to good use. The vision of many in research is for there to be open-access to samples to researchers, with an overseeing governing body that evaluates requests for use to allow access.'
Julie Corfield, Biobanking
To reads the full interviews with these professionals visit the ĎMeet the expertsí page on the U-BIOPRED website. |
|
|
|
U-BIOPRED in the Spotlight |
|
Breda Flood, a person with asthma, member of the project and President of the European Federation of Allergy and Airways Diseases Patientsí Associations (EFA), presented the patientís experience and vision of U-BIOPRED on several occasions. This past March, she presented in Dublin at an EU policy workshop on personalised medicines and public-private partnerships. |
She discussed why a partnership between public and private organisations is needed for severe asthma, and how U-BIOPRED is tackling the challenges involved in developing personalized medicines for asthma. She showcased results so far and provided her thoughts on the reasons for U-BIOPREDís successes, also identifying ways that other scientists researching personalized medicines can learn from the project. |
|
|
|
Working with other projects |
|
U-BIOPRED does not stand alone in the world of respiratory research. We have developed close links with other European projects such as:
- AirPROM, where data from U-BIOPRED is being used to create computer models of human lungs www.airprom.european-lung-foundation.org
- eTRIKS, which is creating a research database which will store U-BIOPRED's data making it easier to compare data from U-BIOPRED and other projects. www.etriks.org
|
- PROactive, which is developing an instrument to help people with chronic obstructive pulmonary disease (COPD) describe their experience of physical activity. www.proactivecopd.com
- SARP, a large ‘sister’ project in the USA whose data can be used to check the results of U-BIOPRED. www.severeasthma.org
|
|
|
|
The U-BIOPRED Exit Questionnaire |
|
An exit questionnaire will be provided to participants in the project upon their final visit to a medical clinic collaborating in U-BIOPRED. If you already have had your final visit to your respective medical clinic, you may find the questionnaire available online here:
https://www.surveymonkey.com/s/U-BIOPRED |
Should you require a version in your local language, translations will be available shortly through the same medical clinic where you were recruited for the project. |
|
|
| Stay in touch |
Tell us your story |
If you would like to continue receiving updates about the project sign up for the U-BIOPRED project newsletter by copy this link into your web browser:
www.ubiopred.european-lung-foundation.org/newsletter |
We would like to hear from people that took part in the project. Your experiences can help us improve the experience of participants in future studies. If you would like to share your story either, e-mail us at ubiopred@europeanlung.org, or visit the webpage for more information
(www.ubiopred.european-lung-foundation.org/your_story). |
|
|
|
|
