The year 2023 was very intense for the development of legislation to address unmet medical needs, ensure better patient access to treatment, to reduce medicines shortages in the EU and be prepared with medical countermeasures against treats to health. EFA collaborated with the EMA and brought the allergy, atopic eczema, asthma and COPD patient perspective and agenda to several European Commission fora.
Shaping a future EU pharmaceutical legislation that works for patients
In 2023, EFA developed our allergy, atopic eczema and respiratory patients asks on the EU proposals to revise the current pharmaceutical framework, one of the most impactful legislations in health at the EU level.
Following one EFA community strategic discussion on the proposals, and after the publication in late April of the European Commission proposals to review the pharmaceutical legislation EFA responded to the two EC final consultations on the Directive on medicines, which emphasises driving innovation and access towards medicines for children and rare disease patients, and the proposal for a Regulation on how medicines are incentivised, authorised, and supervised in the EU.
 As the legislation to be reviewed affects all patients and disease areas in Europe, EFA worked very closely with the European Patients’ Forum (EPF). We contributed to EPF’s ‘paediatric task force’ and the ‘unmet medical needs task force’, sharing in five dedicated meetings the needs and concerns from EFA communities as well as with written contributions which were then published in three EPF’s papers.
As the legislation to be reviewed affects all patients and disease areas in Europe, EFA worked very closely with the European Patients’ Forum (EPF). We contributed to EPF’s ‘paediatric task force’ and the ‘unmet medical needs task force’, sharing in five dedicated meetings the needs and concerns from EFA communities as well as with written contributions which were then published in three EPF’s papers.
EFA’s advocacy on the EU Pharmaceutical legislation in 2023
- May
EFA statement at EC Stakeholder dialogue on the revision of the EU Pharmaceutical legislation
- June
EFA questions to European Commission during a closed EMA/PCWP special webinar on the revision of the pharmaceutical legislation
- July
 A patient-centred revision of the EU pharmaceutical legislation’,
A patient-centred revision of the EU pharmaceutical legislation’, - September
 Ensuring access to paediatric medicines’
Ensuring access to paediatric medicines’ - October
 A patient-centred vision for unmet medical needs’
A patient-centred vision for unmet medical needs’ - November
response to final consultation on the proposal for a Directive on the Union code relating to medicinal products for human use (2023/0132)
- November
response to final consultation on the proposal for a Regulation on laying down procedures for the authorisation and supervision of medicinal products for human use and establishing rules for governing the European Medicines Agency (2023/0131)
EFA coordinated the work of the European Lung Health Group (ELHG) on the revision of the EU pharmaceutical framework, and proposed recommendations to make the proposal more reflective of lung health needs to the European Parliament, that we shared with 13 Members of the European Parliament (MEPs) across all political groups acting as Rapporteurs and Shadow Rapporteurs on the two proposals.

During the EC stakeholder dialogue on the legislative proposals, EFA Director of Policy and Communications Isabel Proaño encouraged the Commission to reinforce the definition of unmet medical needs to reflect disease severity, to maintain the printed package leaflet in medicines, and to cascade information of the novel Environmental Risk Assessment (ERA) to the patient to inform their choices regarding sustainability.
Improving patient-centric authorisation of medicines
Medicines in the EU

The European Medicines Agency (EMA) is requesting more often and deeper collaboration with European level patient organisations, through more opportunities to engage in expert workshops and new tools.
- In 2023, EFA participated in the four EMA Patients’ and Consumers’ Working Party meetings, organised jointly with the Healthcare Professionals Working Party (HPWP).
- Importantly, in 2023, EFA was invited to provide with the community feedback through “early contacts” at the very start of two authorisation application procedures by the Committee on Human Medicinal Products (CHMP): on Ebglyss (lebrikizumab) intended for the treatment of moderate-to-severe atopic dermatitis in adults and adolescents, and on Delgocitinib intended for the treatment of moderate to severe chronic hand eczema.
- A patient from our community participated in November on a Scientific Advisory (SA) procedure evaluation of the benefits and risks on the extension of indication of the pneumococcal vaccine to include infants, children, and adolescents from 6 weeks to less than 18 years of age for the prevention of invasive disease, pneumonia and acute otitis media caused by Streptococcus pneumoniae.
- As a community, EFA ensured the medicine overview reviews by patients for three newly authorised medicines: vaccines to treat Respiratory syncytial virus (RSV) -Abrysvo and Arexvy-, and a medicine treating people against chronic cough (Lyfnua).
- EFA also supported EMA in preparing their communication about a key deliverable of the year: the first Union list of priority medicines, published in December.
- To inform the Pharmacovigilance Risk Assessment Committee (PRAC), we gathered EFA community experience with pholcodine-containing medicines that are indicated in the treatment of non-productive cough to inform EMA’s ongoing safety review for severe allergic reactions and we consulted throughout the year our communities on their awareness of ischaemic cerebrovascular adverse drug reactions with pseudoephedrine-containing medicines.
- We also engaged in regulatory discussions through written consultations to the “EMA Guideline on registry-based studies” and the “Concept paper on revision of the Guideline on clinical investigation of medicinal products in the treatment of patients with acute respiratory distress syndrome”.
- We also brought EFA community experience and views to the high-level discussions around shortages (workshop in March), on the novel qualification methodologies (workshop in April), on access of paediatric patients to clinical trials across borders (workshop from Enpr-EMA project in May), and on the use of big data the value of Real-World Evidence (RWE), novel technologies and methods driving changes in evidence generation (December).
EFA is eligible member organisation to the EMA since 2013, represented by EFA Director of Policy and Communications, Isabel Proaño, and EFA past Vice-President and President of EFA Dutch Member the Stichting Voedselallergie, Erna Botjes.
EFA’s collaboration with EMA in 2023
- 4 PCWP/HPWP meetings
- 2 Early contacts forms submitted to CHMP
- 2 Medicine safety review information to PRAC
- 3 Medicine overviews reviews
- 2 Written consultations on draft guidelines
- 3 Expert workshops
Reducing inequalities by informing EU joint Health Technology Assessment (HTA)
 In 2023, the EU continued preparing the field for the implementation of the EU regulation on Health Technology Assessment (HTAR) adopted end 2021. HTA is the crucial step to assess if and how a medicine will access a market; the assessment informs governments and healthcare systems’ decisions on the eligible patients for a given medicine, the pricing and reimbursement.
In 2023, the EU continued preparing the field for the implementation of the EU regulation on Health Technology Assessment (HTAR) adopted end 2021. HTA is the crucial step to assess if and how a medicine will access a market; the assessment informs governments and healthcare systems’ decisions on the eligible patients for a given medicine, the pricing and reimbursement.
As part of the preparatory work, the European Commission launched the HTA Stakeholder Network and EFA was elected to be a member. The HTA Stakeholder Network is composed of European level patients and healthcare professionals’ associations, research and industry organisations and it will be the central consultative body involved in the upcoming joint work on HTA at EU.
As a vibrant community, in 2023 EFA voiced our community’s expectations for change and evolution in the approach to HTA practices in Europe. We participated in three meetings of the network and expressed the need to increase coherence between clinical trials data, medicines authorization and HTA procedures. We highlighted the absolute need for a shift towards meaningful patient involvement on HTA and to avoid the current tokenism. We also encouraged the Commission to develop future-proof procedures that embrace upcoming medicinal technologies, especially combination products (medicine with a medical device) and e-products.

From left to right, Isabel Proaño (Director of Policy and Communications at EFA), Maya Mathews (European Commission Head of Unit of Unit in charge of HTA at DG SANTE), Julie Spony (Policy Officer at European Patients’ Forum) and Rosa Castro (Senior Policy Manager at European Public Health Alliance) during the first meeting of the HTA Stakeholder network in June.
EFA’s perspective will guide the Commission proposals for adjacent legislation on the HTAR foreseen to be adopted in 2024. EFA will continue bringing these issues up towards 2030, the moment when joint EU HTA will also be applicable to technologies for allergy and airways diseases.
Advocating for safer and cleaner medicines
As for the sustainable transition of the pharmaceutical sector, in September EFA participated in the European Chemicals Agency (ECHA) consultation on the proposal for the restriction of certain per- and polyfluoroalkyl substances (PFASs). Currently, there are several PFAS for medical use which are used as excipients in rescue inhalers for asthma and COPD. The ECHA restriction proposal has been brought up by several Member States and it is grounded on concerns over the persistence of PFASs on the environment after being used and released, and the subsequent risks for public health.
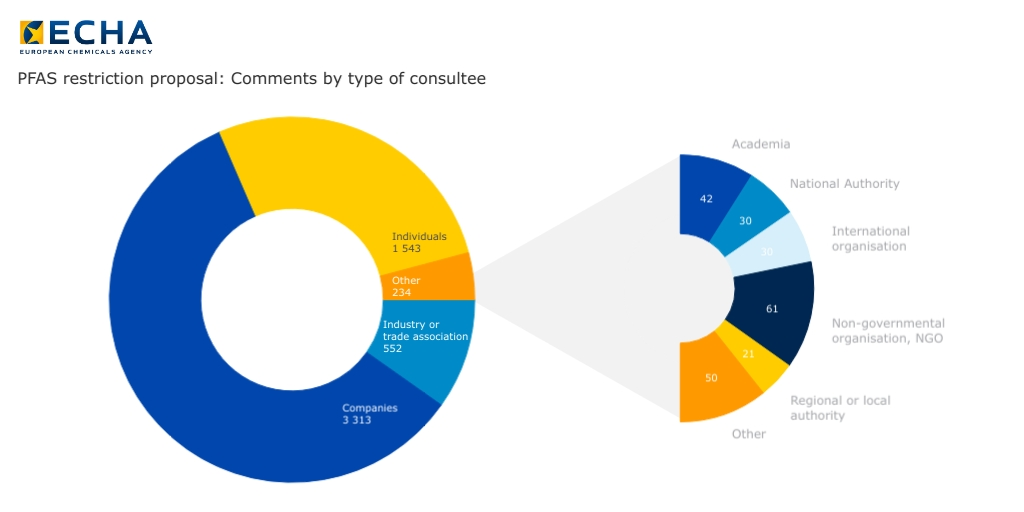
As representatives of people who depend on PFAS containing medicines, and at the same time are impacted by their use, EFA put forward recommendations for more scientifically valid information and foresight on how an EU restriction would affect access to basic medication for asthma and COPD patients. EFA also recommended to consider investing in patient education, to optimise the use of PFAS containing medicines, and providing more information about the medicines’ composition, environmental footprint, its presentation, and disposal options.
Health Data, breaking digital barriers for patients
Following the launch of a proposal for a Regulation on a European Health Data Space (EHDS) in 2022, EFA continued its work and reach out to the Members of the European Parliament in 2023 to put forward patients’ views to the sharing of digital health data.
 We held two meetings with key Members of the European Parliament (MEPs) from S&D, MEP Petar Vitanov (Bulgaria), Shadow Rapporteur on EHDS at the LIBE Committee, and from Renew, MEP Andreas Gluck (Germany), Shadow Rapporteur on EHDS Opinion at the ITRE Committee. Moreover, EFA has contributed to and taken part in the European Patients’ Forum (EPF) Digital Health working group who met three times in 2023. Together and supporting EPF, we have shown strong alignment on important patient considerations arising from the EHDS proposal, such as the need to have and ‘opt-out mechanism’ when collecting individual data and sharing it, and the premises for patients and organisations to have ‘access to justice’ in case of data use misconduct.
We held two meetings with key Members of the European Parliament (MEPs) from S&D, MEP Petar Vitanov (Bulgaria), Shadow Rapporteur on EHDS at the LIBE Committee, and from Renew, MEP Andreas Gluck (Germany), Shadow Rapporteur on EHDS Opinion at the ITRE Committee. Moreover, EFA has contributed to and taken part in the European Patients’ Forum (EPF) Digital Health working group who met three times in 2023. Together and supporting EPF, we have shown strong alignment on important patient considerations arising from the EHDS proposal, such as the need to have and ‘opt-out mechanism’ when collecting individual data and sharing it, and the premises for patients and organisations to have ‘access to justice’ in case of data use misconduct.
 In 2023, as the Joint Action Towards a European Health Data Space (TEHDAS) wrapped up its work, EFA attended the final Stakeholder Forum in June, which served to present the final recommendations on data governance, infrastructure, and citizens’ views on data use. EFA contributed to TEHDAS with input to the recommendations, reinforcing patients’ involvement in data governance. Specifically, EFA together with the European Lung Health Group highlighted that in the future European Health Data Space (EHDS), patients should not become health data subjects, but rather be at the centre of the digital health ecosystem. With strengthened and clear communication, transparency, and accountability in data sharing, there will also be strengthened trust and value.
In 2023, as the Joint Action Towards a European Health Data Space (TEHDAS) wrapped up its work, EFA attended the final Stakeholder Forum in June, which served to present the final recommendations on data governance, infrastructure, and citizens’ views on data use. EFA contributed to TEHDAS with input to the recommendations, reinforcing patients’ involvement in data governance. Specifically, EFA together with the European Lung Health Group highlighted that in the future European Health Data Space (EHDS), patients should not become health data subjects, but rather be at the centre of the digital health ecosystem. With strengthened and clear communication, transparency, and accountability in data sharing, there will also be strengthened trust and value.
 EFA also continued its partnership with the Healthcare and Information Management System Society (HIMSS), being one of the few organizations representing patients’ views on health data. In 2023, EFA was selected as speaker in one of the main sessions of the HIMSS European Health Conference & Exhibition in Lisbon, discussing patients and data. With this occasion, EFA Vice-President, Armando Ruiz brought forward ‘Patients & health data: Insights from respiratory diseases patients’, on key trust issues for patients when accessing health data across borders and within regional healthcare systems.
EFA also continued its partnership with the Healthcare and Information Management System Society (HIMSS), being one of the few organizations representing patients’ views on health data. In 2023, EFA was selected as speaker in one of the main sessions of the HIMSS European Health Conference & Exhibition in Lisbon, discussing patients and data. With this occasion, EFA Vice-President, Armando Ruiz brought forward ‘Patients & health data: Insights from respiratory diseases patients’, on key trust issues for patients when accessing health data across borders and within regional healthcare systems.

EFA Vice-President, Armando Ruiz, brought the patient respiratory perspective to health data and digitalisation to the HIMSS 2023 European conference.
Disease prevention
Following EFA’s election to the Civil Society Forum (CSF) of the European Commission Health Emergency Preparedness and Response Authority (HERA) in 2022, EFA actively participated informing the work of HERA in 2023, attending all meetings and contributing to the consultations and participating the three ad hoc working groups (future of HERA, health threats, and training). EFA actively contributed to the CSF discussion paper on the strategy and future role of HERA, strongly defending the role of civil society in emergency response as well as the need to broaden the scope of HERA to also consider non-communicable diseases such as allergy and respiratory diseases on preparedness and response, and not just infectious threats.

In 2023, EFA participated in three different workstreams of HERA bringing EFA’s community perspective: the prioritisation exercise of medical countermeasures against health threats (i.e., medicines, vaccines, medical devices), the optimal requirements for pandemic-proof respiratory protection technologies, and the horizon scanning of technologies to address pathogens in indoor air.
As member of the CSF, EFA brought the patient perspective hand in hand with the European Patients Forum (EPF) to the annual HERA 2023 conference as the one of the two civil society representatives in the panels. Our intervention on addressing medicines shortages, which included an example or a shortage affecting basic and critical asthma treatment, was appreciated by the co-panellists and audience.

EFA’s Director of Policy and Communications Isabel Proaño (on the left) brought the patient to the HERA Conference session on ‘Ensuring the availability of critical medical countermeasures in Europe’ with EMA Executive Director Emer Cooke, Portuguese Minister of Health Manuel Pizarro, MEP Sara Cerdas (Portugal/S&D) and HERA Director General Laurent Muschel.